In vivo Study of a Self-stabilizing Artificial Vertebral Body Fabricated by Electron Beam Melting
2015-01-08 文章来源:Peking University Third Hospital Jun Yang, Hong Cai, Jia 我要说
Introduction
Various pathologies can lead to anterior column defects and spine instability, such as tumors, fractures, and infections . Vertebral body replacement (VBR) through an anterior approach is a widely accepted procedure for restoring the anterior column height.
Electron beam melting (EBM) is a three—dimensional (3-D) printing technology that can, using metal powder, produce porous metal implants with a particular shape and pore structure. Previous studies suggest that porous metal fabricated by EBM was biocompatible and allowed osteogenesis. To date, research on an artificial vertebral body fabricated by EBM has, to our knowledge, not been reported. Thus, we sought to investigate the feasibility of a novel artificial vertebral body without an additional anterior plate or a posterior internal fixation, otherwise known as a self—stabilizing artificial vertebral body (SSAVB) fabricated by EBM in a sheep model.
Materials and methods
First, 16 adult male Small Tail Han Sheep were purchased. Each sheep underwent C4—subtotal corpectomy with posterior vertebral wall retention at approximately one—third of the vertebral body depth and SSAVB implantation. All sheep were divided into two groups (n = 8). Group A was observed for 6 weeks before sacrifice, and group B was observed for 12 weeks. All sheep received post—surgical X-rays and were sacrificed respectively to assess implant location. Four specimens of each group weresubjected to micro—CT followed by histological examination, and another 4 specimens were harvested for biomechanical testing.
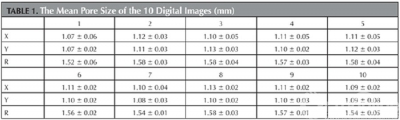
SSAVB size and shape were designed according to the radiological measurements of each sheep cervical spine prior to surgery to produce an individualized implant. A scanning electron microscope (SEM) was used to study the micro—structural surface characteristics and 10 digital images were randomly selected. For each digital image, 3 dimensional values of the pore were selected and the average dimension was calculated.10 samples of the same size were used for compression and Young’s modulus tests. Compression tests were performed using a materials testing machine (MTS 858 Bionix machine, MTS System Inc., Minneapolis, MN) with a loading rate of 1 mm/min. Young’s modulus was calculated as the slope of the straight—line portion of the stress-strain curve.
There were 4 groups in the biomechanical test: Group A was the in vivo group observed for 6 weeks; group B was the in vivo group observed for 12 weeks; group C, which was the control group did not have SSAVB implantation (intact C2 to C6 segment, n = 4); group D, the in vitro group consisted of 4 fresh sheep cervical spines from C2 to C6 segment which underwent C4-subtotal corpectomy with the posterior vertebral wall retention and SSAVB implantation (n = 4).
The range of motion (ROM) of the C3—C5 of intact and SSAVB—implanted sheep spines were compared with respect to kinematic behavior. ROM was defined as the angular displacement during minimum and maximum bending moment.
Results
Fig 1a-1 depicts a SEM image of the SSAVB surface microstructure. Table 1 depicts the mean samples pore size .
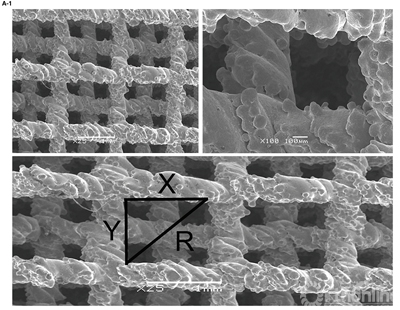
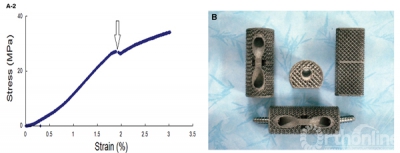
Figure 1.A-1 , SEM image of the implant surface and method of measuring pore size.A-2, Implant strain—stress curve, the arrow indicates trabecular structure fractures under corresponding compressive strength.B shows the implant product. SEM indicates scanning electron microscope.
The compressive strength and Young’s modulus were 26.8 MPa and 12.9 GPa respectively. The mean compressive strength and Young’s modulus of the 10 samples were 27.61 ± 1.21 MPa and 14.24 ± 1.11 GPa, respectively. Fig 1b shows the product of SSAVB.
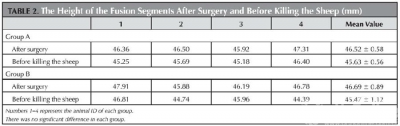
Fig 2a depicts the x-ray films from group A and B.Table 2 depicts the height of the fusion segments after surgery and before sacrifice, there was no significant difference in each group (p = 0.07 and 0.14 respectively).
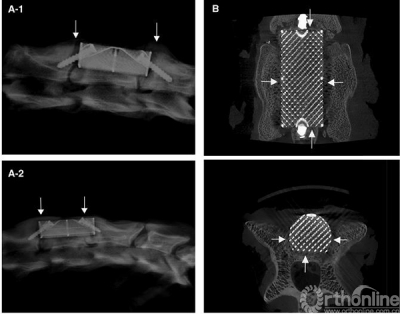
Figure 2:
Lateral radiographic film before sacrifice of group A (Figure 2a-1) and group B (Figure 2a-2). Gaps exist between the SSAVB and C4 posterior vertebral wall as well as C3 and C5 vertebral body which became fuzzy at the 6th week and almost disappeared at the 12th week, arrows indicate the growth of bone bridge. From the Micro—CT image of the implant (Figure 1b) we can observe the bone ingrowth.
We observed the bone ingrowth directly through Micro—CT. Fig 2b shows that the bone ingrowth from posterior vertebral wall and both sides of the vertebral body can be observed.
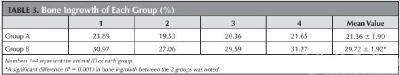
Bone ingrowth was observed from the posterior vertebral wall and adjacent vertebral body (Fig 3a and b). Differences in the percentage of bone ingrowth between group A (21.36% ± 1.90%) and group B (29.72% ± 1.92%) are depicted in table 3, and these differences were significant between the two groups (p = 0.001).
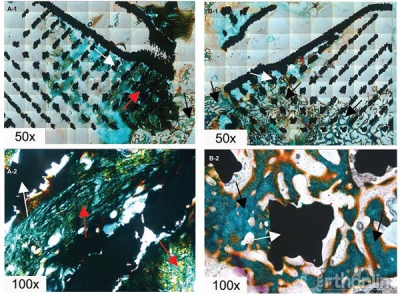
Figure 3:
Histological analysis of bone tissue growth into porous implant of group A (Figure 3a-1 and a-2) and group B (Figure 3b-1 and b-2). White arrows, red arrows and black arrows indicate the metal, the immature bone tissue and the mature bone tissue respectively.
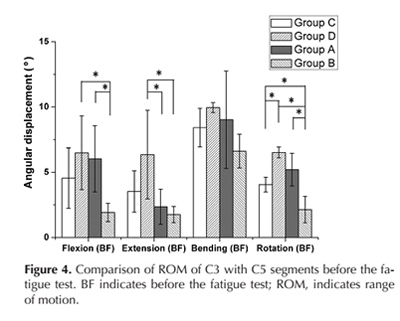
Before the fatigue test, there was a significant difference in the ROM of flexion between group D and B (p = 0.038), A and B (p = 0.04), and in the ROM of extension between group D and A (p = 0.041), D and B (p = 0.041). Additionally, there was a significant difference in the ROM of rotation between group C and D (p = 0.009), C and B (p = 0.027), D and B (p = 0.000), A and B (p = 0.003) (Fig 4).
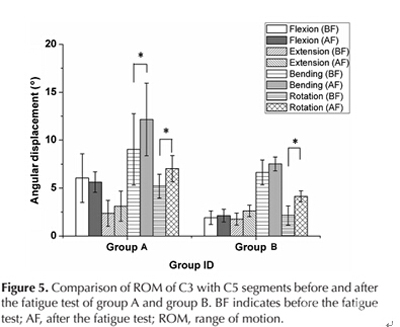
After the fatigue test, there was a significant difference in the ROM of flexion between group A and B (p = 0.031). In group A, there was a significant difference in the ROM of bending (p = 0.03) and rotation (p = 0.027) before and after fatigue test. In group B, there was a significant difference in the ROM of rotation (p = 0.04) before and after the fatigue test (Fig 5).
Discussion
In this study, we investigated the feasibility of using a self—stabilizing artificial vertebral body fabricated by EBM for vertebral body replacement. The SSAVB rough surface was formed by the melting Ti6Al4V particles. According to Xue’s study, a rough surface will not only enhance cell adhesion, but also stimulate cell differentiation. Successful cell adhesion is necessary for bone ingrowth, and our sample had excellent bone ingrowth.
Mismatch between Young’s modulus of the implant and that of the host bone can lead to a stress shielding effect which will eventually cause the implant to fail. The stress—strain curve (Fig 1a-2) displays the mechanical properties of the SSAVB. The arrow indicates that under this stress (26.8 MPa) some trabecular metal fractured. The mechanical property of what was similar to cancellous bone, and it should minimize the stress shielding effect. Parthasarathy and co—workers reported that the strength of the porous structure depends on the dimensions of the struts and the porosity. However, after bone grows into the implant, it becomes a “ferroconcrete structure”, and compressive strength is significantly increased. Therefore, Young’s modulus may be more important because it plays a key role in early bone fusion.
We can observe that the height of the fusion segments decreased, however, there was no significant difference. Furthermore, the cylindrical shape of them do not provide good contact between the implant and host bone. In addition, conventional cages often need anterior plate fixation which may lead to an esophageal fistula or other complications. The SSAVB fabricated by EBM has a flat posterior wall and two ends that are in close contact with the posterior vertebral wall and endplate. The in vivo experiment revealed that, throughout the whole experiment the implant did not collapse, subsidence or displacement, and the implant had close contact with host bone enabling bone to grow from the host bone bed.
We performed the in vitro implantation (Group D) ROM test to ensure the immediate stability of the SSAVB. Data show that although four—direction ROM (flexion, extension, bending, and rotation) was greater than that of the control group (Group C), there was a significant difference only in the rotation ROM. This difference may be related to the method of fixation. The bilateral facet was not destroyed, however, there was only one screw in each end. To enhance the immediate rotation stability, two screws can be inserted into each end.
For in vivo groups, the ROM of group A in three directions (flexion, bending and rotation) was increased compared with group C, however, there was no significant difference between the two groups. Furthermore, after 12 weeks, the ROM in all four directions decreased, especially with respect to rotation, and this trend indicated that early stability depended on screws and fibrous tissue. After bone grew into the implant, stability was enhanced.
After the fatigue test, we observed that the ROM of the in vivo groups increased. Moreover, the ROM of group A with respect to flexion, bending, and rotation was greater than that of group C, however, there was no significant difference between two groups. In addition, the ROM in all four directions of group B was lower than that of group C, indicating that after 12 weeks, the newly formed bone was sufficient for maintaining implant stability.
The study confirms the feasibility of using the novel a artificial vertebral body fabricated by EBM for vertebral body replacement. X-ray radiophotograph evidence reveals no fracture or displacement of the implant when used this way. Micro-CT and histological assessment provide direct evidence that bone tissues grow into the porous implant structure. Biomechanical test conformed that the implant can maintain cervical spine stability.


 京公网安备11010502051256号
京公网安备11010502051256号